EQUIOXX INJECTION 20MG/ML (firocoxib) has been the only coxib non-steroidal anti-inflammatory drug (NSAID) approved for use in horses. Now, this once-daily treatment to control joint pain and inflammation associated with equine osteoarthritis, is approved by the FDA in tablet form, and it will be available in the market early fall._x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_nEQUIOXX is the only NSAID approved for use up to 14 days by both the AQHA and USEF1,2† and now gives the choice of three formulations, depending on the specific need of the horse. Injection is best to initiate therapy, while the Paste is a convenient form for accurate dosing, especially for small horses and performance horses. And now, Tablets are easy to administer, with or without feed._x000D_n_x000D_n“Veterinarians and horse owners have been waiting for a tablet option o from Merial,” said Hoyt Cheramie, DVM, MS, Manager, Merial Large Animal Veterinary Services._x000D_n_x000D_n“The FDA approval process is very stringent, and we are proud to bring this new tablet formulation to market for horses. Having all three formulations allows for consistent therapy with the same active ingredient.”_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n“When a horse needs relief from osteoarthritis at home or at a show, owners should partner with their veterinarians to determine the best treatment options, be diligent about choosing the right formulations, and follow dosing directions,” said Cheramie. He noted that when it comes to giving medications to performance horses, owners, trainers and veterinarians should read the rules specific to each association or show, ensuring they are in compliance._x000D_n_x000D_nEQUIOXX Tablets will be available by prescription only. Veterinarians are encouraged to reach out to their Merial Sales Representatives to get more details about the upcoming availability of this new formulation._x000D_n_x000D_nIMPORTANT SAFETY INFORMATION: As with any prescription medication, prior to use, a veterinarian should perform a physical examination and review the horse’s medical history. A veterinarian should advise horse owners to observe for signs of potential drug toxicity. As a class, nonsteroidal anti-inflammatory drugs may be associated with gastrointestinal, hepatic and renal toxicity. Use with other NSAIDs, corticosteroids or nephrotoxic medication should be avoided. EQUIOXX has not been tested in horses less than 1 year of age or in breeding horses, or pregnant or lactating mares. For additional information, please refer to the prescribing information or visit_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_nAbout Merial_x000D_n_x000D_nMerial is a world-leading, innovation-driven animal health company, providing a comprehensive range of products that focus on disease prevention and overall health and wellness in animals. Merial has three main business areas: pets, farm animals, and veterinary public health, and our health solutions target more than 200 diseases and conditions across a variety of species. Merial employs 6,900 people and operates in more than 150 countries worldwide with over €2.5 billion of sales in 2015. Merial is a Sanofi company. For more information, please see_x000D_n_x000D_n† EQUIOXX Injection may be used for five of the 14 days._x000D_n_x000D_n1 American Quarter Horse Association. Conditionally permitted therapeutic medications._x000D_n_x000D_nOfficial Handbook of Rules and Regulations. 2016:45-48._x000D_n_x000D_n2 United States Equestrian Federation. Guidelines for Drugs and Medications. 2015:8-9._x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_nNew FDA-Approved Generic Drug NexHA Now Available to Treat Joint Dysfunction in Horses_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_nKindred Biosciences Receives FDA Approval of Zimeta™ (dipyrone injection) for the Control of Pyrexia in Horses_x000D_nZimeta was approved for the control of fever in horses._x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_nroduces New RealPCR Diagnostics for Safeguarding Equine Health_x000D_nNew diagnostics enable early diagnosis of critical equine diseases to help mitigate health risks for patients and financial risks for ownershttp://www.tacomavetmedication.com_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_nLiability With Use of Non-FDA-Approved Drugs_x000D_nMost equine practitioners are diligent about following standards of care when treating equine patients. But what happens when a horse experiences an adverse event after an off-label or compounded medication is used?_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_nFDA Approves First Generic Ketoprofen for Use in Horses_x000D_nThe FDA has approved KetoMed, the first generic ketoprofen for the alleviation of inflammation and pain associated with musculoskeletal disorders in horses._x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_nBI Receives European Commission Approval for Aservo EquiHaler_x000D_nThis week, the European Commission granted marketing authorization to Boehringer Ingelheim for the new for horses that suffer from severe equine asthma._x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n_x000D_n
Sale!
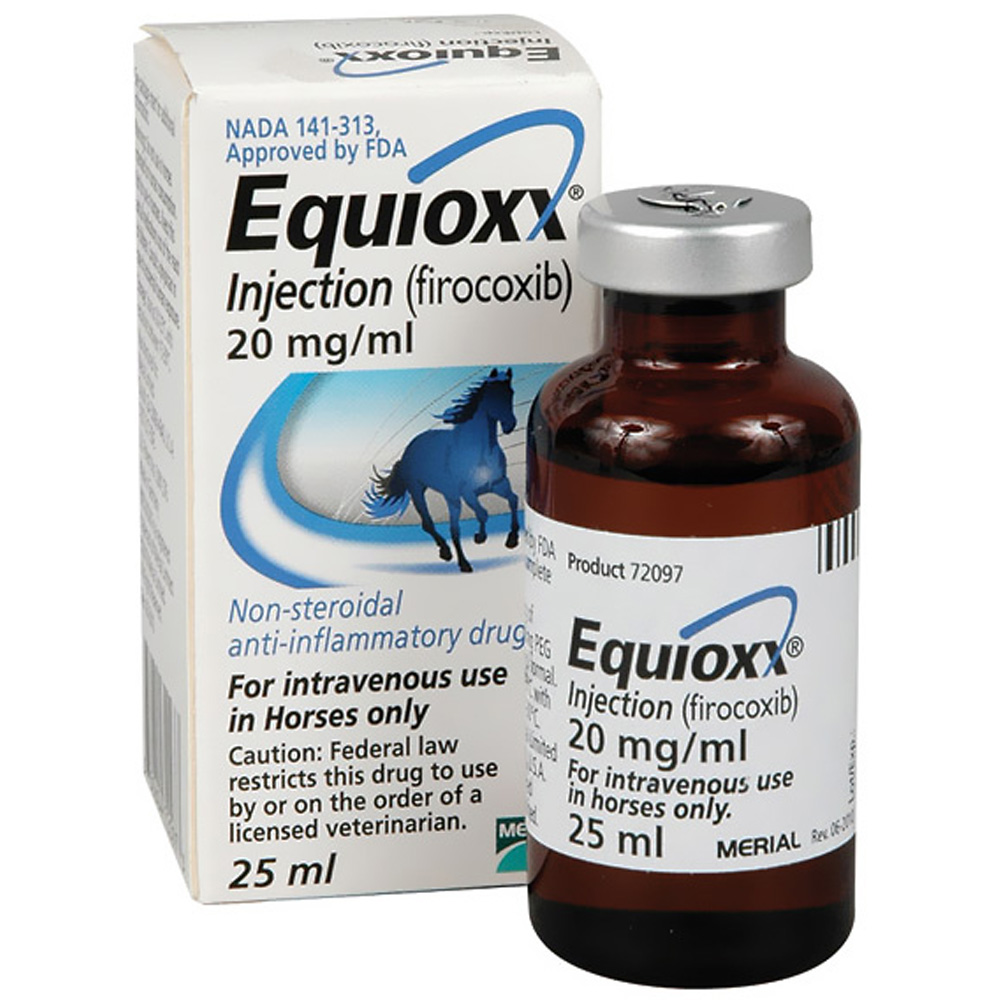
EQUIOXX INJECTION 20MG/ML
$75.00
EQUIOXX (firocoxib) has been the only coxib non-steroidal anti-inflammatory drug (NSAID) approved for use in horses. Now, this once-daily treatment to control joint pain and




Reviews
There are no reviews yet.